Development pipeline
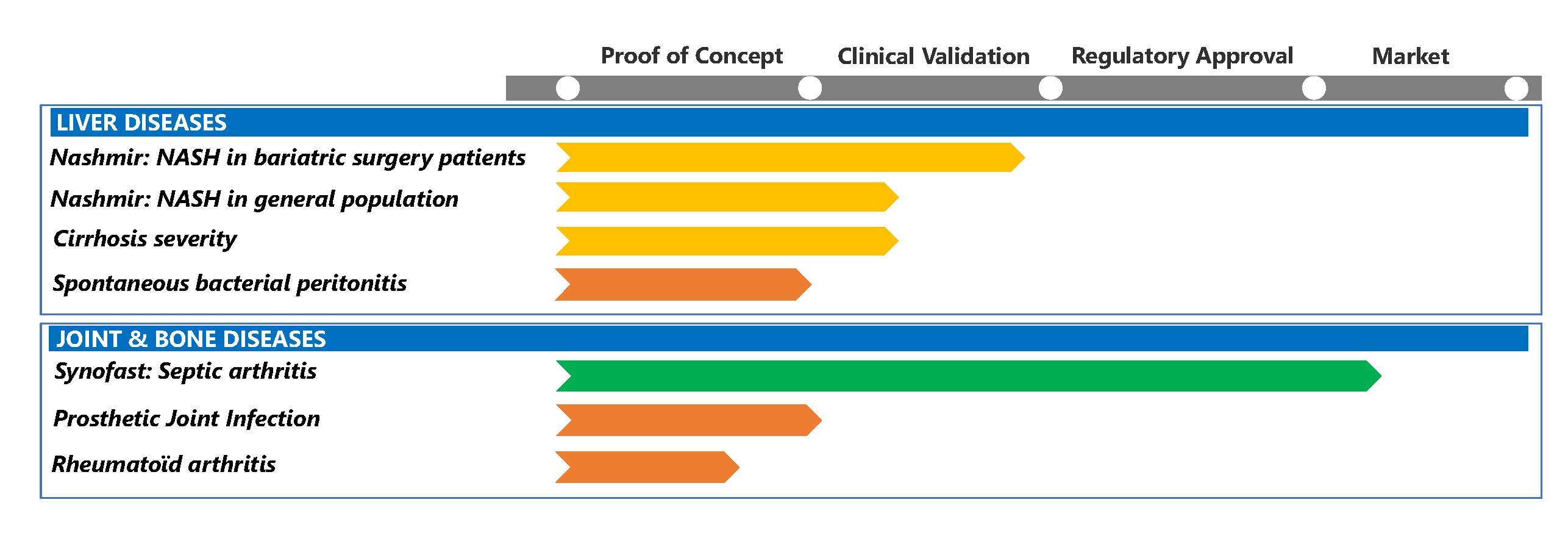
NASHMIR™
Non-Alcoholic Steato-Hepatitis (or NASH) is the most common chronic liver disorder in Western countries. It is a possible outcome of metabolic disorders such as obesity or type 2 diabetes. It is estimated that more than 5% of the population worldwide is affected by NASH, which main consequences are cirrhosis and hepato-cellular carcinoma.
Today, histological examination of the liver through a biopsy is required to diagnose NASH. Hence, the development of new non-invasive methods is a need recognized by health authorities, necessary for an improved patient care.
DIAFIR, develops NASHMIR®, a non-invasive test based on the metabolic signature of NASH, from a simple drop of serum, which aim to replace the current invasive procedure. Our patented technology combines the mid-infrared light blood serum recording and the machine learning algorithm diagnosis score delivery.
SYNOFAST™

Acute septic arthritis is an inflammation of the synovial membrane of the joint resulting from an infection. The rapid progress of the pathology (within a few hours to a few days maximum) can lead to irreversible destruction of the joint tissues and even mortality in 10% of the cases in the event of a diagnostic delay, making septic arthritis a therapeutic emergency.
Although septic arthritis is rare, its management cost is significant: It requires a 48 hours minimum hospitalization for preventive antibiotic therapy, pending the results of the diagnosis based on the cytology of synovial fluid.
Diafir develops SYNOFAST®, a rapid diagnostic orientation test of septic arthritis cases, based on the mid-infrared spectroscopic analysis of the synovial fluid, allowing sepsis detection in less than 10 minutes. The result is a rapid management of patients at risk and avoid unnecessary hospitalization of the one which are not, and therefore a significant cost saving for health authorities.


